Driven by a passion to solve scientific challenges
Our vision is to become the leading provider of primary cell culture solutions to enable life-saving therapies.
We are here to enable your stem cell vision
BioLamina is a biotechnology company built on a scientific foundation with a legacy in matrix biology and cell culture-based research. We want to help scientists who have struggled for decades to culture embryonic stem cells, induced pluripotent stem cells, and other primary cells. By providing tools for efficient and easy culture, BioLamina aims to smooth the path towards safe and effective cell therapy. Whether your goal is to answer basic scientific questions, advance cell therapies, or make models for drug development, our products can help you in your journey. The power of our Biolaminin® substrates has been shown in numerous publications and they will continue to provide vital support for the stem cell community – from scientific concepts to clinical studies.

Founded to make cell therapy a reality
It all started back in 2008. Kristian Tryggvason, with a Ph.D. in molecular biology and just having finished MBA studies, sat with his father Karl Tryggvason, Professor at Karolinska Institute, to listen to a business proposal. Karl had a research focus on extracellular matrix proteins, and after years of unsuccessful attempts by many groups, he had finally been able to produce full-length, human recombinant laminin proteins. From those first discoveries, Karl understood that he had invented an excellent research tool with the potential to facilitate the development of cell therapies. BioLamina was founded and quite right; during the company’s first ten years, the full-length Biolaminin products have proved to be essential for high-quality cell research and cell therapy development.
For us, knowledge is everything. We want to be recognized for our premium products, competence, and service. Being an accountable partner, both towards colleagues and customers, is central in our organization. We do what we say we will do, and we do it with our hearts.
Management Team

Veronica Byfield Sköld
Chief Executive Officer
Learn more
Professional experience
Veronica has an extensive experience from the broader healthcare and life science field, with a focus on strategy, business development, and commercialization in highly regulated markets. She began her career at management consulting firm The Boston Consulting Group (BCG, and has since held several management positions in companies like Gambro (renal care and blood & cell component technology), Elekta (radiation oncology, radiosurgery, and neuroscience), and Permobil (advanced rehab), heading up activities within Strategy, Marketing, Commercialization, and Medical Affairs in global organizations. Prior to joining BioLamina in 2021, she also spent four years as an investment partner at the Nordic healthcare investment firm Impilo.
Education
- MSc in Industrial Engineering and Management from Lund University, Sweden and the University of California, USA

Therése Kallur, PhD
Chief Scientific Officer &
Vice President Business Development
Learn more
Professional experience
Therése is a multilateral life science leader and a key member at BioLamina since its beginning. She has consistently worked in roles combining scientific knowledge and commercial aptitude such as within Business Development, Product Management, Marketing and Sales. Prior to her work at BioLamina, Therése worked a couple of years as a post-doc at Max-Planck-Institute for Neurological Research in Cologne, Germany.
Education
- PhD in Neurobiology, Lund University, Sweden
- MSc in Molecular biology, Lund University, Sweden
- BSc in Psychology, Lund University, Sweden

Ulrika Ljungkvist
Chief Operations Officer
Learn more
Professional experience
Ulrika is an experienced life science leader with a demonstrated history of senior management in the pharmaceutical and biotechnology industry. Before working as BioLamina, she was a consultant in Life Science Operations/CMC, Leadership & Project Management. She previously worked as Site Manager at CDMO Cobra Biologics (mamalian cell) and as Head of Projects & Process Technology at Recipharm Oral Solid Dosages site for Antibiotics. Prior to this experience, she worked at AstraZeneca on various positions such as Head of API Process Technology (small molecules) and Head of Pilot Plant AZ Bio PR&D (biologics API).
Education
- MSc, Chemical Engineering/Biochemistry, KTH Royal Institute of Technology, Stockholm, Sweden and University of Sydney, Australia
- Business Certificate in Manufacturing Excellence, University of Warwick, UK

Harald Eriksson, PhD
Vice President Quality
Learn more
Professional experience
Harald has a strong experience in Quality and Management in the pharmaceutical and biological industry. Prior to his current position, Harald has been Head of Quality and he previously worked at AstraZeneca, with the last position as Manager QA Microbiology. In parallel, Harald successively hold different management board positions for the city of Stockholm, both in public companies and city boards.
Education
- Ph.D., Molecular Genetics, Stockholm University, Sweden
- M.Sc. Molecular Biology, Stockholm University, Sweden
- M.Sc. Molecular Life Sciences, Stockholm University, Sweden
- B.Sc. Molecular Biology, Stockholm University, Sweden

Mattias Gäreskog
Chief Commercial Officer
Learn more
Professional experience
Mattias has a wealth of management experience from the commercial field. Prior to his current position, he served as Business Development Manager within BioLamina. He has more than 15 years in commercial roles at Mercodia AB, as Managing Director Marketing & Sales, acting CEO, Global Sales Manager, Scientific Sales manager and Product Specialist and Product Manager. He has also experience in recruitment, as a consultant and Market Area Manager at Clockwork Bemanning & Rekrytering.
Mattias hold his post-doc position at Uppsala University and performed his research in the field of diabetes and associated complications.
Education
- PhD in Medical Cell Biology, Uppsala University, Sweden
- MSc in Biomedicine, Uppsala University, Sweden

Anders Lindblad
VP Strategic Development
Learn more
Professional experience
Anders has a long array of experience in Life Science industry in general and with BioLamina in particular. Joining the company in 2011 as Project Manager, he has since held many roles within the company. Prior to his current position as VP Strategic Development, other roles held at BioLamina include COO and interim CEO.
Experience outside of BioLamina include working as independent consultant for pharmaceutical and medical device industries, holding the position of COO at Cadila Pharmaceuticals Sweden, and working at both Anocca and SentoClone (Quality Manager and Project Manager) with setup of new laboratories and QMS for cell-based medicinal products.
Education
- M.Sc. in Industrial Engineering and Business Management, KTH Royal Institute of Technology, Stockholm, Sweden

Lotta Larsson
Head of People
Learn more
Professional experience
Lotta Larsson has a solid 15-year experience of establishing and managing a global HR function in a fast-growing, international and life science environment. Before joining BioLamina, she was the Global HR Manager of the MedTech company RaySearch Laboratories AB.
Education
- Bachelors of Applied Science, Health Sciences and Health Promotion, University of Deakin, Australia
- Bachelor of Applied Science, Dietetics and Clinical Nutrition, University of Deakin, Australia

Magnus Åkerhielm
Chief Financial Officer
Learn more
Professional experience
Magnus has more than 25 years of experience in finance and management, from different sectors and businesses.
Prior to his current position at BioLamina, he worked as Managing Director for Nytida, the largest business area within the listed Nordic healthcare group Ambea. He has also been the Managing Director of Keaolis Sverige. He has previously been CFO at ISS Facility Services, M2 Engineering and Spendrups Bryggeri.
Education
- BSc in Finance, Stockholm School of Economics, Sweden
- MBA in General Management, Darden School of Business, University of Virginia, USA
Board Members

Sophie Hagströmer
Chairperson of the board
Learn more
Current position
Investment Director at Bure Equity AB
Chairman of the Board Allgon AB
Board member Bure Growth AB
Professional experience
Bure is an investment company that has been listed on the Nasdaq Stockholm Large Cap stock exchange since 1993. The portfolio consists of listed and unlisted companies. Bure has a long-term investment horizon and the ownership philosophy is based on a deep commitment and high presence in the portfolio companies.
Prior to joining Bure Equity, Sophie has held various investment management positions including Partner at Scope Equity as well as Senior Investment Manager at Novax. Previous board assignments include, inter alia, Board member of Colosseum Smile, Footbalance, Skruvat.se, Brand Factory, Artificial Solutions, Lagerhaus and DesignTorget (Chair). Sophie also has experience from Corporate Finance and Management Consulting.
Education
- MSc in Industrial Engineering and Management, Lund University, Sweden and the University of California, USA

Max Jonson
Board member
Learn more
Current position
CFO at Bure Equity AB
Professional experience
Prior joining Bure Equity, Max has been the CFO of IFL at the Stockholm School of Economics (2012–2013), the CFO of Orasolv AB (publ.) (2011–2012) and dpnova AB (2009–2011) and worked in Corporate Finance and Leveraged Finance at Kaupthing Bank (2003– 2009) and Corporate Finance at SEB (1996–2003).
Education
- Stockholm School of Economics, Sweden
- BSc in Economics and International Business, New York University, USA
- MBA in Analytic Finance, University of Chicago, USA

Jonas Pålsson
Board member
Learn more
Current position
Co-Founder of the Investment company Northislet
Professional experience
Northislet is an active investor in private and public, small and midsized growth companies, primarily within the Life Science industry in the Nordic region. Prior to co-founding Northislet, Jonas spend 13 years as a Managing Director and Partner with the global hedge fund Eton Park, managing the fund’s public equity investments in Europe and Asia
Education
- MSc in Economics and Business Administration, Stockholm School of Economics, Sweden

Kristian Tryggvason
Board member
Learn more
Current position
Founder and CEO of Alder Therapeutics
Professional experience
Prior to Alder, Kristian founded BioLamina. He has also held business development and project management positions in the pharmaceutical sector, where he created patent strategies, out-licensed therapy products, and gained broad exposure to the clinical, regulatory, and commercial aspects of drug development
Education
- MSc in Molecular Biology, University of Oulu, Finland,
- Ph.D. in Cellular and Molecular Biology, Karolinska Institutet, Sweden
- MBA, Copenhagen Business School, Denmark

Lotta Ljungqvist
Board member
Learn more
Current position
Board member of NorthXBiologics, AroCell, BioArctic AB, Genovis Aktiebolag, Atlas Antibodies AB, SwedenBio Service AB and 4L Bioconsulting AB.
Professional experience
Passionate about Life Sciences, Lotta has an extensive experience of managing global technology businesses and development of biopharmaceutical companies.
She was head of R&D BioProcess GE Life Sciences at GE Healthcare. In 2007/2008, she was the CEO of IMEA AB, a biotechnology company that develops biological drugs. Prior to that, she was responsible for the Biopharmaceutical Contract Manufacturing Organization within Biovitrum AB.
Education
- PhD in Biochemical Engineering, KTH Royal Institute of Technology, Sweden

Catarina Flyborg
Board Member
Learn more
Current position
Strategic Advisor at Cytiva
Board member of CCRM
Professional experience
Over her 25-year career in the biotechnology industry, Catarina has held various management positions in sales, marketing, communications and product development. She worrked at Cytiva, as VP of the Cell & Gene Therapy. She held various positions at GE Healthcare Life Sciences (previous name of Cytiva): General Manager Commercial and Marketing Cell Therapy, General Manager BioProcess Products, Leader Enterprise Solutions,
Leader Vaccine Initiative, Head of Communication.
Education
- MSc in Chemistry, Chalmers University of Technology, Sweden
- MSc in Biochemistry, KTH Royal Institute of Technology, Sweden

Alex Slack
Board Member
Learn more
Current position
Founding Partner at Lauxera Capital Partners
Professional experience
Alex is a healthcare specialist investor.
After starting his career as a management consultant at McKinsey & Company in Boston, he joined Maverick Capital’s healthcare team. Alex focused on long/short public equity and venture capital/cross-over investments, ultimately as Managing Director. Alex then joined Jackson Square Partners where he focused on high-conviction healthcare public equities and led the firm’s ESG activities.
Education
- AB History, magna cum laude, Phi Beta Kappa, Harvard University, USA
Key shareholders
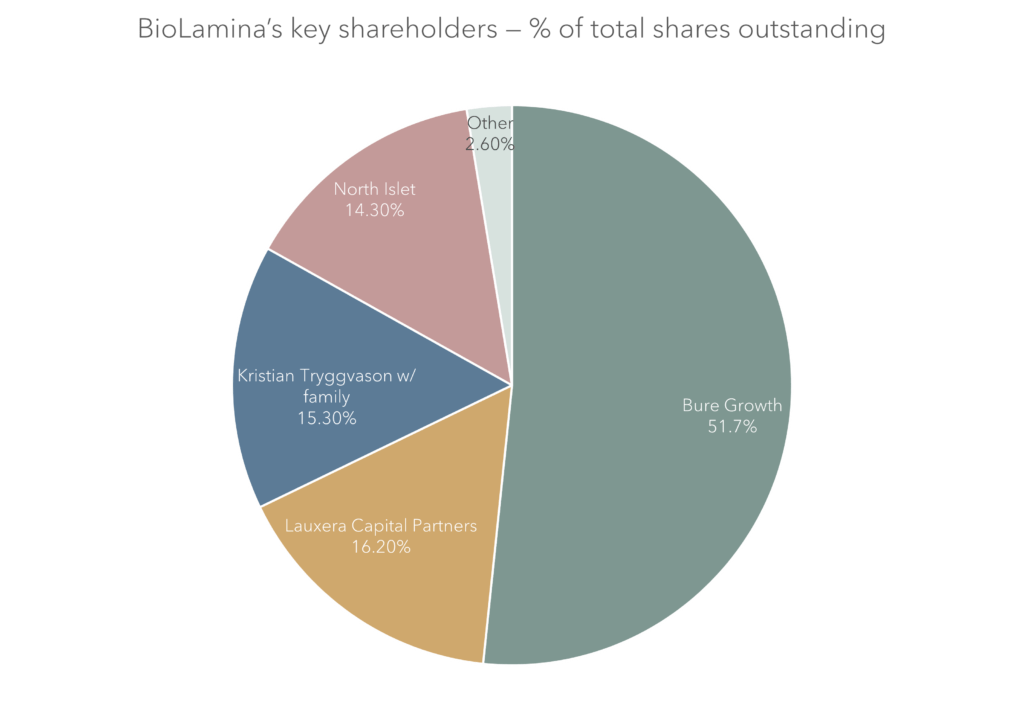
| Shareholder | % of total shares outstanding |
| Bure Growth | 51.7% |
| Lauxera | 16.2% |
| Kristian Tryggvason w/ family | 15.3% |
| North Islet | 14.3% |
| Other | 2.6% |
| Total | 100,0% |

A desire to contribute to the stem cell community

The highly educated, international team at BioLamina is characterized by scientific curiosity. Personal responsibility and accountability are the foundations of our company, as is a high level of collaboration, both within the company and with our partners. Our team is highly diversified and international and we uphold the Nordic standards of equality and partnership in the way we develop both products and collaborations. We like to do things together, as equals. This means that creativity and innovation always trump hierarchy and norm and that we celebrate joint efforts in our strive to contribute to the stem cell community and solve scientific challenges.
WHAT OUR EMPLOYEES SAY
“Working together, building on each other’s ideas and knowledge, creates greatness. I am proud that we are unpretentious, curious, and fearless enough to dare to approach and talk to people often more talented than we to build new partnerships. These abilities have helped us to find new ways forward through collaborations.”

Therése Kallur, PhD
CSO, VP Business Development
Revolutionize cell culture with us
To work in partnership is a way to reach new innovations. Our goal is to solve challenges in the cell culture field by learning from our partners and evolving together with them. We enjoy discussing cutting-edge science and meeting talented people with their unique knowledge and scientific views. We want to build sustainable relationships with researchers from a myriad of backgrounds and with a variety of skills.
For the projects we engage in, our contributions can be found in all parts of the project development process: from planning, writing project plans, project managing, and reporting, to executing tasks with deliverables related to cell culture improvements and culture standardization. Currently, more than one-third of our product development projects are funded via public grants and for half of these, we are the lead project manager. All public-funded projects involve at least three collaborators working together, in order to bring our overarching vision of enabling cell therapy to fruition.
We love collaborations and working with ambitious people who are driven by a clear purpose. Together we can achieve something greater!
Contact our Business Development to discuss your project.

We participate in different projects funded by:


Talk to our team for customized support
We are here to help you in your journey.